Researchers have developed a new battery technology that could help create batteries that last longer than previously thought possible.
A team of researchers at the US Department of Energy's Oak Ridge National Laboratory (ORNL) have challenged a long-held assumption that a battery's three main components - the positive cathode, negative anode and ion-conducting electrolyte - can play only one role each in the device.
The electrolyte in the team's new battery design has dual functions: it serves not only as an ion conductor but also as a cathode supplement.
This cooperative chemistry, enabled by the use of an ORNL-developed solid electrolyte, delivers an extra boost to the battery's capacity and extends the lifespan of the device.
"This bi-functional electrolyte revolutionises the concept of conventional batteries and opens a new avenue for the design of batteries with unprecedented energy density," said ORNL's Chengdu Liang.
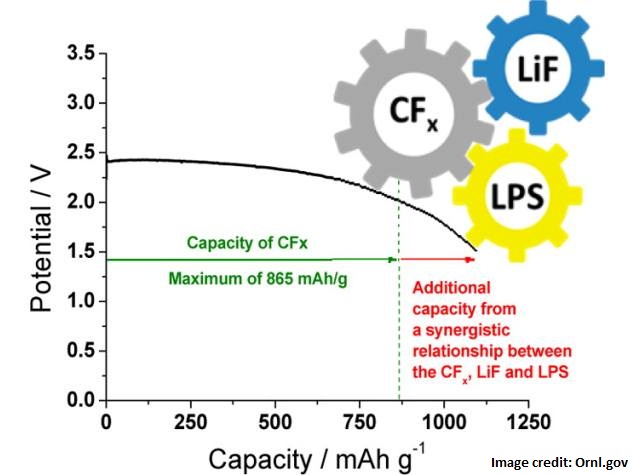
The team demonstrated the new concept in a lithium carbon fluoride battery, considered one of the best single-use batteries because of its high energy density, stability and long shelf life.
When ORNL researchers incorporated a solid lithium thiophosphate electrolyte, the battery generated a 26 percent higher capacity than what would be its theoretical maximum if each component acted independently.
The increase, said Liang, is caused by the cooperative interactions between the electrolyte and cathode.
"As the battery discharges, it generates a lithium fluoride salt that further catalyses the electrochemical activity of the electrolyte," Liang said.
"This relationship converts the electrolyte - conventionally an inactive component in capacity - to an active one," he said.
The improvement in capacity could translate into years or even decades of extra life, depending on how the battery is engineered and used.
Longer-lived disposable batteries are in demand for applications such as such as artificial cardiac pacemakers, radiofrequency identification devices, remote keyless system, and sensors, where replacing or recharging a battery is not possible or desirable.
"If you have a pacemaker, you don't want to undergo surgery every 10 years to replace the battery," Liang said.
"What if a battery could last 30 to 50 years? Our fundamental research is opening up that possibility through a new design mechanism," Liang added.
The study is published in the Journal of the American Chemical Society.
No comments:
Post a Comment